The Importance of Choosing a CANNZ Practitioner for Your Cosmetic Treatments – A Guide to Safe and Effective Care
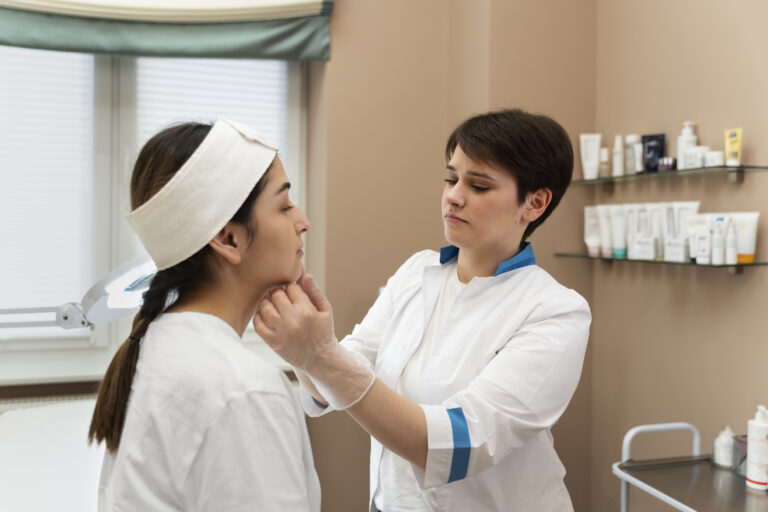
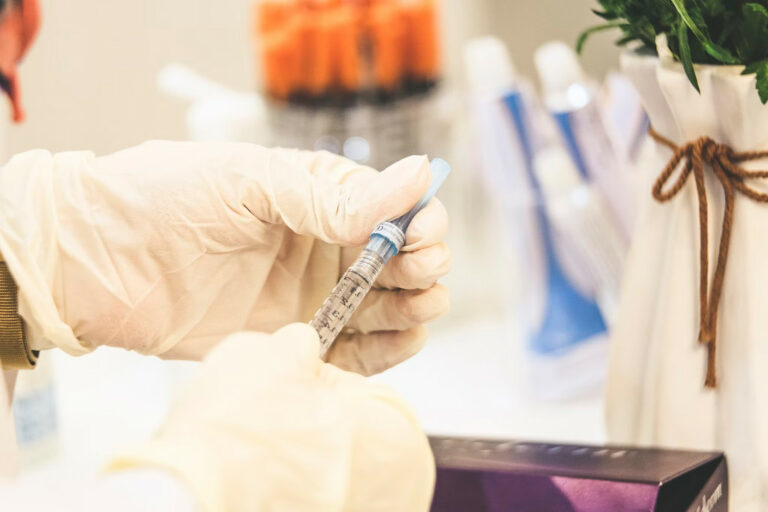
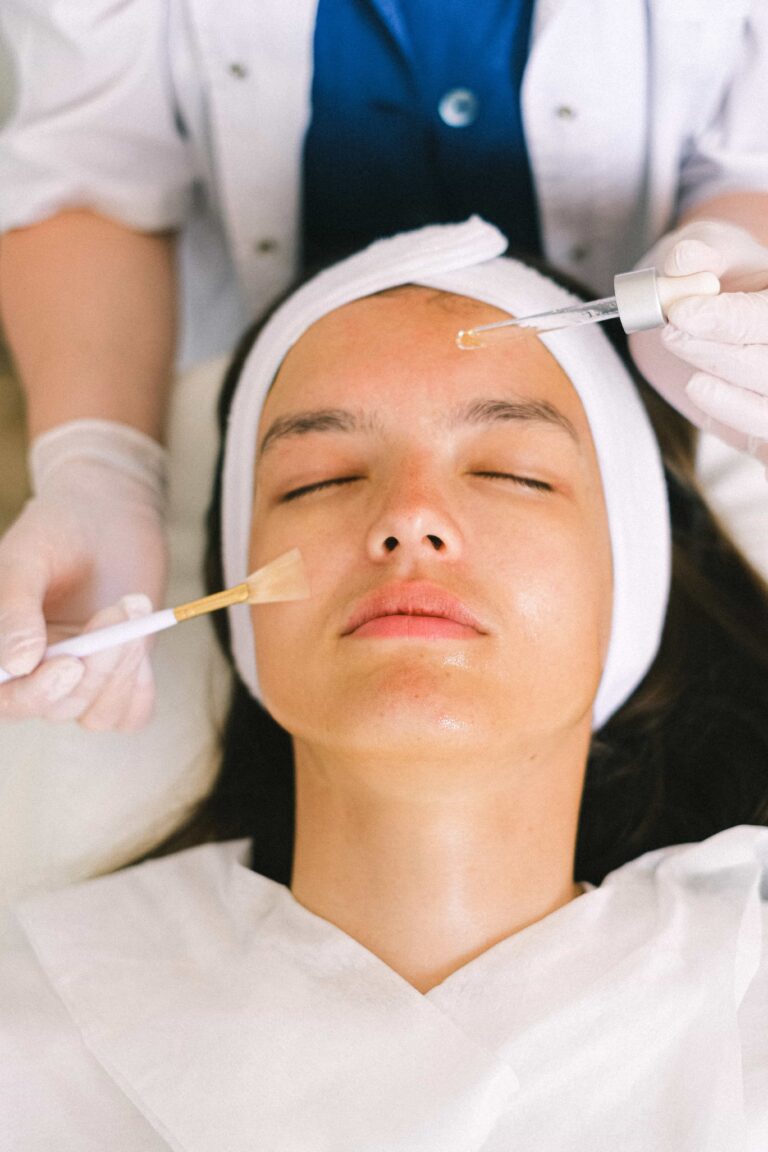
Article at a glance Selecting the right practitioner when enhancing your natural beauty through cosmetic treatments is paramount. In recent years, the demand for cosmetic procedures has surged, leading to an influx of providers in the industry. However, not all…